Though human gut microbiota does not represent the main research area of our group, we can deal with such topic in a collaboration. A new paper on gut microbiota of autistic and healthy children is just one example. There were significant differences in gut microbiota of these two groups of children although it is difficult to decide whether this differences in gut microbiota were a cause or consequence of autism. We prefer the latter hypothesis, i.e. that changes in gut microbiota of autistic children are consequence and not cause of specific patterns in behavior of autistic children which may include particular food preference. However, even in this case, there is no reason why not to consider interventions that may lead to restauration of “autistic” microbiota to healthy one, being aware of the fact that this will likely be of rather small consequence for total behavioral status of autistic children.
Author Archive: Ivan Rychlik
New paper on metabolism of selected anaerobes in the chicken caecum
A new paper on the metabolism of 20 anaerobic bacteria in the caecum of chickens has been published in June 2024. Different species of genus Bacteroides degraded (poly)saccharides. Bacteroides mediterraneensis also expressed type VI secrection system which is used by bacteria for inactivation of competing bacterial species in mixed microbial populations. E. coli or Succinatimonas laso preferred carbohydrate fermentation and the same was true also for Bifidobacterium although carbohydrate fermentation followed a pathways slightly different from reference glycolysis. Megamonas hypermegale and Megamonas funiformis were less dependent on carbohydrate ferementation than previous bacterial species and Megamonas complemented their carbohydrate metabolism by aminoacid degradation. Campylobacter, Sutterella and Phascolarctobacterium degraded aminoacids nearly exclusively with minimal dependence on carbohydrates. Campylobacter expressed flagellar proteins and was therefore motile when colonising chicken caecum. This information can be used for desigh of specific probiotic mixtures competing with particular pathogens not only in the intestinal tract of chickens byt also other farm animals or humans.
New probiotic product QuoCNA
On April 2, 2024, we received decision from the Institute for State Control of Veterinary Biologicals and Medicines which permits free distribution of new probiotic product QuoCNA in the Czech Republic.
Product QuoCNA is based on results of own research in the area of gut microbiota of chickens lasting for more than 10 years. Product consists of 9 strict anaerobes which originate and efficiently colonise chicken caecum (for composition see package leaflet in Czech language). QuoCNA is intended for rapid and safe colonisation of the caecum of both meat and egg lines chickens, of newly hatched chicks in particular. Considering gut microbiology, the product replaces contact of chicks with adult hen which is interrupted when hatching in hatcheries. Administration of QuoCNA results in the development of natural gut microbiota in newly hatched chickens which would happened if the chicks were hatched in nests and remained in contact with adult hen and whole flock. Such colonisation is known to lead to suppression of gut colonisation with enteric pathognes like Salmonella. QuoCNA can be used also for the restoration of gut microbiota in older age categories of chickens, e.g. after antibiotic therapy. Performance of QuoCNA is similar to the competitive exclusion products from which it differs by the fact that its compostion is clearly defined. We have shown that administration of QuoCNA decreases abundance of pathogenic E. coli in intestinal tract and unpublished data show that it increases chicken resistance to Salmonella infection by a factor of 50.
How to identify bacterial horizontally transmissible genes including those responsible for antibiotic resistance
Identification of horizontally transmissible genes including those responsible for antibiotic resistance is apparently a single task. However, immediately after it turns out that this task is not that simple. In our new paper in the Microbiology Spectrum focused on this issue and proposed a functional pipeline. Our conclusions can be founded in this pdf file.
New paper on importance of contact between adult hen and newly hatched chicks for the development of skin and respiratory tract microbiota
A new paper from our production has been accepted for publication in the Poultry Science. This paper introduces microbial composition of skin and tracheal microbiota and further develops importance of contact between a hen and newly hatched chicks for the development of skin and respiratory tract microbiota of chickens. Many bacteria of gut origin were detected as a part of skin and trachea microbiota but since we used DNA sequencing to determine microbiota composition, this was likely only a DNA from no longer viable gut anaerobes. On the other hand, Lactobacilli and Streptococci belonged among likely vital bacteria which were common in skin and tracheal microbiota of chicks in contact with adult hens. Cutibacterium acnes represented another taxon common in skin and respiratory tract microbiota. Finally, Clostridium disporicum and Clostridium perfringens were common in skin microbiota but only rarely entered respiratory tract.
New publication on association of gut microbiota and metabolite composition in the caecum of one week old chickens
Our new paper on the relationship of gut microbiota and presence of low molecular weight compounds in caecal digesta of one-week-old chickens has been just published in the Poultry Science. A general conclusion is that colonisation of the chicken caecum with Bacteroides, Megamonas, Megasphaera, Phascolarctobacterium, Succinatimonas or Sutterella (gut anaerobes) leads to release of additional molecules of plant origin from the feed into digesta. Digesta of chicks colonised by gut anaerobes was enriched for derivates of nucleotides and amino acids, and products of their fermentation. Digesta of chicks colonised by gut anaerobes was enriched also for biologically active molecules like daidzein, genistein, glycitein, nicotinamide, feruloyl putrescine or feruloyl agmatine. There were also some exceptions confirming this rule, e.g. glutamate and tyramine were more abundant in caecal digesta of control chickens. Similarly, betaine or soyasaponin I dominated in digesta of control chickens in comparison to those colonised by gut anaerobe mixture.
We are still considering placement of this knowledge since increased abundance of a given metabolite is a compromise between degradative function of gut microbiota of feed ingredients associated with release of additional molecules, metabolic function of gut microbiota and their ability to ferment just released amino acids or nucleotides, ability of chickens to resorb newly available nutrients and ability of chickens to secrete their own metabolites in caecal digesta. Increased abundance of daidzein, genistein or glycitein, all soya flavonoids, therefore indicates inability of chickens to resorb them in such an amount. On the other hand, disappearance of betaine in digesta of chicks colonised by gut anaerobe mixture indicates that at least one of them can utilise and degrade betaine. Despite such uncertainties, a mere fact that the composition of gut microbiota extensively modifies composition of low molecular weight compounds means that it is possible to enrich digesta for biologically active molecules by a combination of feed formula modification and administration of defined mixtures of selected gut microbiota members.
What is the origin of this or that bacterial species?
We have updated our file on the occurrence of different bacteria in different hosts. This file was enriched for new data on the distribution of bacterial genera and species along chicken production. Once you find out how to work with this file, you will see that Anaerostipes is tightly associated with humans, Succinatimonas with chickens and Phascolarctobacterium represent genus representatives of which can be found in intestinal tract of different hosts. In chicken production, Brevibacterium and Brachybacterium are associated with litter, Acinetobacter with eggshell waste and Cutibacterium with skin and trachea. However, you can find out much more information, e.g. on the distribution of Lactobacilli in humans or dogs. Download our file for more information here.
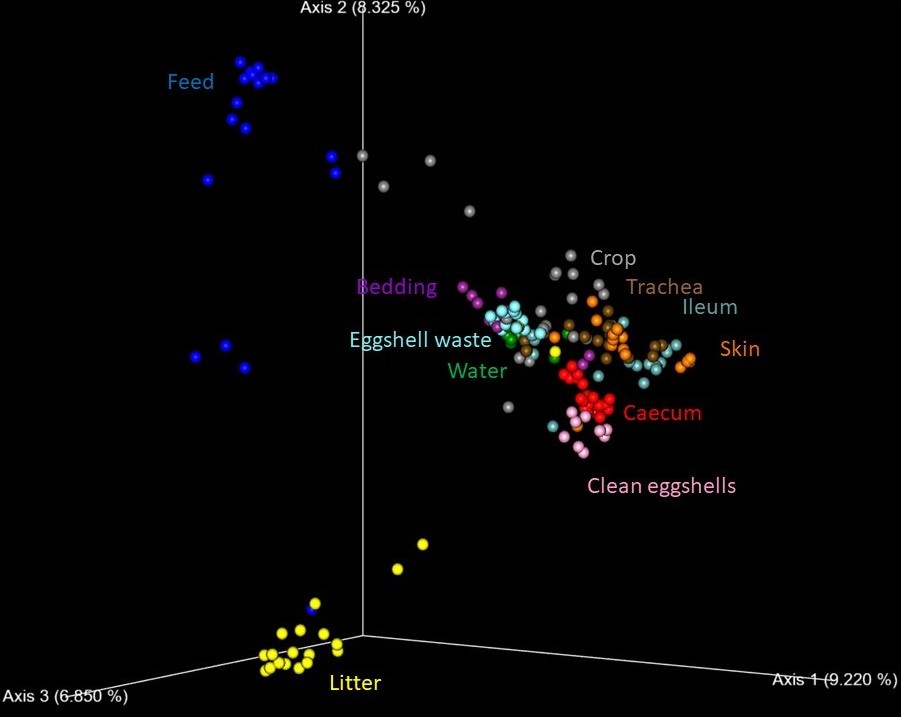
New paper on microbiota of chickens and their environment – review in Avian Diseases, January 2023
A new review paper from our production has been accepted for publication in the Avian Diseases. This review introduces bacterial flora in different chicken organs as well as in their environment. As is the case in our reviews, we have decided for less standard and rather challenging review in which we mention alternative views and challenging associations. The challenging approach is not a purpose for itself. Instead, reason for such style is to force readers to think, to agree (better possibility) or to disagree, to get readers out of their daily comfort of routine way of thinking. One example of alternative idea presented in the review is the information that E. coli is not a species adapted to intestinal tract. Instead, E. coli dominates in chicken environment from where it enters intestinal tract. There are more alternative ideas in this review. If you are ready to think differently, find the original paper here.
Probiotic mixture for protection of chickens against pathogenic E. coli – new publication January 2023
New paper on the protection of chickens by defined probiotic mixtures consisting of strict gut anaerobes against pathogenic E. coli has been just accepted for publication in the Poultry Science. Although we successfully test different defined mixtures of strict gut anaerobes in a protection of chickens against Salmonella, the first published paper reports on the viability of such concept in a protection of chickens against pathogenic E. coli. Currently we prepare the first product of this type for registration in the Czech Republic in 2023, and this will be followed by its commercial distribution immediately after. Information on the ability to suppress infection by pathogenic E. coli can be found here.

New paper on litter microbiota in chicken production
A new paper on the development of litter microbiota in chicken production has been accepted in the Applied and Environmental Microbiology in which we describe a time-dependent development. Escherichia coli, Staphylococcus saprophyticus, and Weissella jogaejeotgali were characteristic of fresh litter during the first month of production. Corynebacterium casei and Lactobacilli dominated in a 2-month-old litter, Brevibacterium, Brachybacterium, and Sphingobacterium were characteristic for 3-month-old litter, and Salinococcus, Dietzia, Yaniella, and Staphylococcus lentus were common in a 4-month-old litter. Identification of the species present in the litter opens the possibility for active manipulation of litter microbiota, something what we are already working on.
